THE CRUNCH GROUP
Division of Applied Mathematics
Brown University
Providence, RI 02912, USA


Funding NIH - R01HL094270 |
||
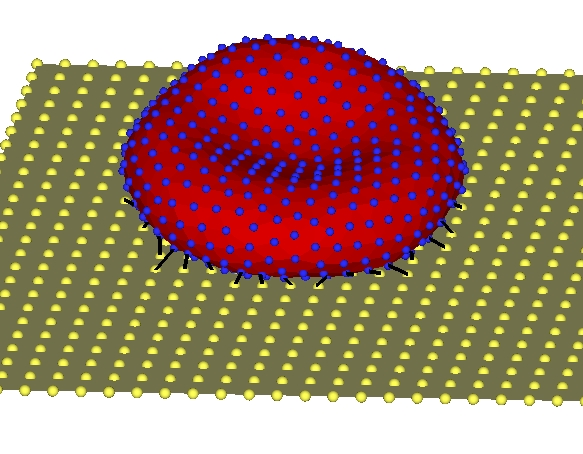
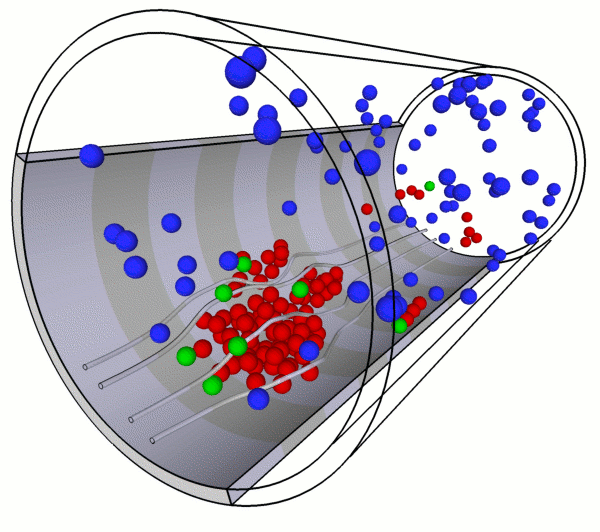
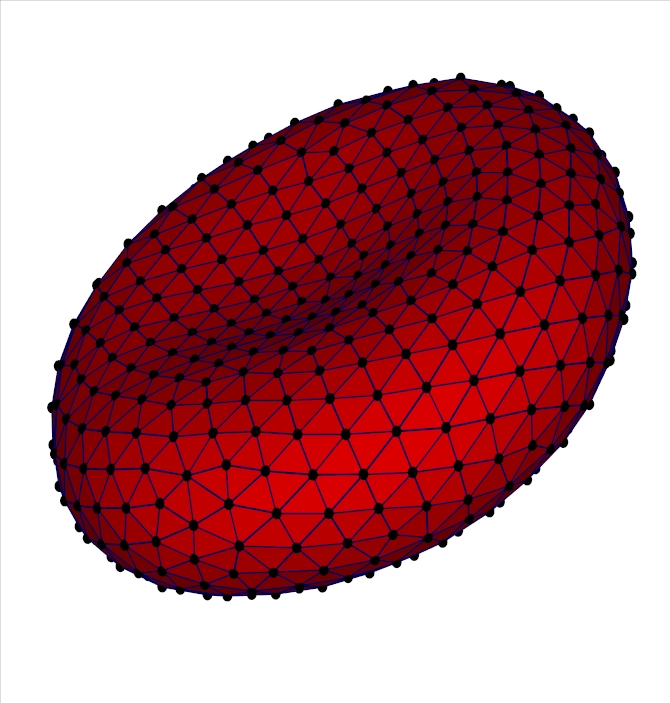
Multiscale Modeling and Parallel Simulations of Blood Flow in Cerebral Malaria and Sickle Cell DiseasesThe objective of this project is to develop a unified and validated multiscale modeling methodology for two diseases with serious hematological disorders: cerebral malaria (CM) and sickle-cell (SS) anemia. The common clinical symptom of both diseases is obstruction in the microcirculation caused primarily by loss of deformability of red blood cells (RBCs) and increased cytoadhesion. Both diseases are characterized by multiscale phenomena, spanning at least four orders of magnitude in length scale with corresponding disparity in the temporal scale. Moreover, the local vaso-oclusions occurring in CM and SS strongly affect blood flow and oxygen transport at the global organ scale as well. Building on recent progress in modeling RBCs at the spectrin level and cell-aggregation processes and taking advantage of available petaflop-level computing resources, we propose a parallel multiscale methodology to model CM and SS and use it as a predictive tool for quantitatively assessing the severity of these diseases. This will form a general simulation platform for adding further complexity in future studies, e.g., incorporating more biochemical details or studying other hemolytic disorders. Predictability of multiscale models requires quantifying uncertainty, and, to this end, we will incorporate polynomial chaos methods to model and propagate parametric uncertainties through the multiscale system. In addition, to validate the new methodology, microfluidic experiments, optical tweezers measurements and 3D phase microscopy will be used to test different aspects of the conceptual and numerical modeling under different conditions. The specific contributions of this project include: (1) Development of fine- and coarse-grained RBC models in CM (cytoskeleton dynamics) and SS (oxygen transport and polymerization) using molecular dynamics (MD), partial differential equations (PDEs), and mean-field theory. (2) Characterization of infected RBCs and sickle cells at different developmental stages using optical non-invasive means. (3) Modeling of flow and rheology in small vessels. Flow modeling will be based on the "triple-decker" - a new algorithm that we have developed for interfacing seamlessly MD, mesoscopic dynamics, and the Navier-Stokes equations. For mesoscopic dynamics we will employ the dissipative particle dynamics (DPD) method, a particularly effective simulation approach for complex fluids. We plan to disseminate our models and software tools, including the general-purpose triple-decker algorithm, via web-based repositories, existing public openware sites, summer schools, and through the MSM consortium. |
||
Publications
|
||
FundingNIH - R01HL094270 |